Brain-CODE: Welcome to Your New Workspace
Brain-CODE is a one-stop shop for data management, storage, analysis and sharing for researchers in neuroscience or interested in brain disorder related data. It is a web-based platform that offers many tools to handle your data in one convenient portal. Using Brain-CODE as your new workspace will help facilitate effective and efficient data sharing with other researchers.
Once data is entered, researchers can both manage and analyze their data using the portal’s toolset. Brain-CODE provides customizable study dashboards which provide a visual overview of your study to showcase key variables and help you manage your study.
Brain-CODE is hosted at the Centre for Advanced Computing, a high performance computing facility which maximizes the security of data but also provides expansive computing resources for complex data analysis. Brain-CODE provides researchers with common analysis tools but also lets them use their own software licenses to perform analyses in virtual workspaces. That way, researchers can access all data made available for sharing on Brain-CODE in a powerful and secure analytics environment.
Learn more about Brain-CODE's tools and features in the following video:
Using Brain-CODE
Brain-CODE's Zone-Based Infrastructure
OBI has designed a highly secure, zone-based infrastructure for Brain-CODE (Figure 2). The three zone structure is advantageous from both a privacy and security perspective as it permits functional separation of sensitive data, ensuring that it is only available to authorized users. The intent is to maximize data sharing potential while minimizing risk to participant privacy.
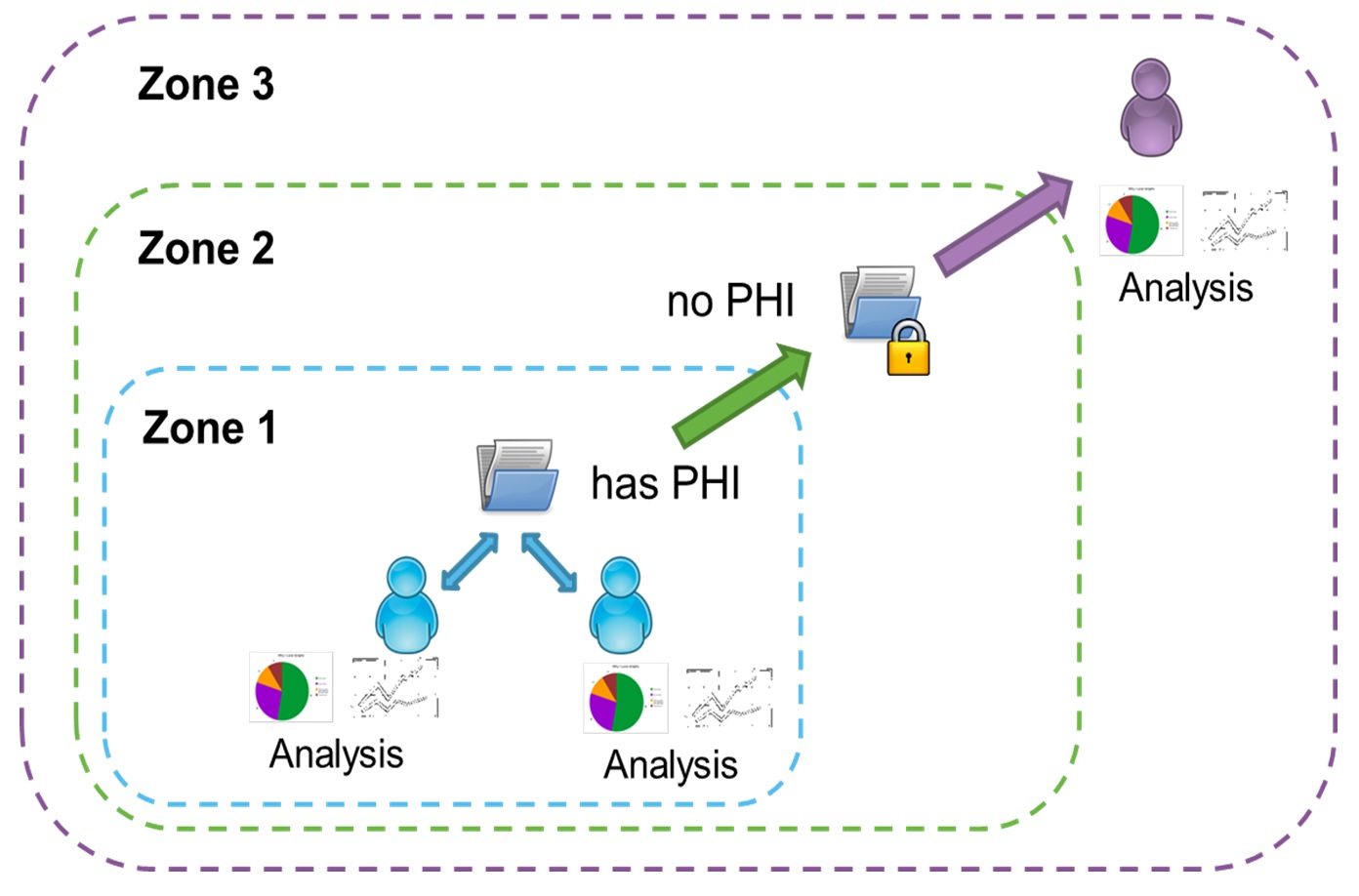
Figure 2. Brain-CODE’s zone-based infrastructure. Three zones exist with distinct purposes and data access parameters to maximize data sharing while minimizing risk to participant privacy and their personal health information (PHI).
Zone 1: Data Management & Analysis
Data uploaded to Brain-CODE will first be entered into Zone 1. Zone 1 allows the management, analysis and sharing of data with direct collaborators. Data in Zone 1 may include Personal Health Information (PHI) that has been approved for upload by your REB and stated in the study participant consent form. Data producers will have full control over the data provided in Zone 1.
After establishing an exclusivity plan with OBI, access to data that you have access to in Zone 1 will be exclusive to you and your direct collaborators for a period of 12 months; this is called your exclusivity period. After the exclusivity period, you will continue to have full access to data in Zone 1. At all times, Brain-CODE can provide you with access to a suite of analytical tools and workspaces.
Zone 2: Long-Term Storage of De-identified Data
Zone 2 provides long-term storage of the datasets. Data in Zone 2 will be labelled as either Public Data, or Controlled Data. Public Data are data from either basic science or that contain no PHI and can be shared directly with Brain-CODE users. Controlled Data are data that have been de-identified, a process which removes direct identifiers using the most advanced tools available. More rarely, Raw Data may be placed in Brain-CODE for long-term storage purposes, in each case as authorized by the Study Protocol and applicable REB and will also be labelled as Controlled Data.
This zone is also where your data can be augmented through links to external databases in a secure environment. The ability for linkages by encrypted health card numbers or probabilistic matching is being developed. Following any data linkage, de-identification tools will be applied to the newly-expanded datasets to ensure the risk of individual participant re-identification is minimized.
Zone 3: Interface for Third-Party Data Access
Zone 3 is the interface for third-party users to access data stored in Zone 2. Third-party users will be able to search and browse information about Public and Controlled data records. While Public data can be accessed directly by users, Controlled data will require users to submit Data Access Requests. Data Access requests are reviewed by Brain-CODE’s Data Access Committee which is composed of researchers, neuroinformatics experts, and OBI staff. Once Data Access Requests are approved, third-party users are given access to data for retrieval and analyses. Prior to releases, requested datasets will undergo are-identification risk analysis to ensure participant privacy.
Requested datasets can be exported to an analytics workspace that are available to all registered Brain-CODE users upon request. A customized workspace can be created to allow access to high performance computing and analysis tools.
To obtain information on Brain-CODE security and privacy measures, or to report any concerns, please e-mail help@braincode.ca
Data Management
Defining Data on Brain-CODE
Two types of data canbe entered into Brain-CODE:
1) Raw Data: Data that have not been modified from their original state or processed to remove any direct identifiers, and may include PHI
- This applies to data that OBI collects from a Participating Institution in order to process it on their behalf, and to data that will be placed in Zone 2 of Brain-CODE that cannot readily have Direct Identifiers removed due to technical limitations (e.g. possible for genomics and MRI data
2) Processed Data: These data have been proecssed, either by OBI or through its Service Group or locally by the Participating Institution, to remove direct identifiers of an individual study participant
- This applies to the majority of data that will be placed in Zone 2 of Brain-CODE.
Two types of data are defined for access on Brain-CODE in Zone 3:
1) Public Data: Data that do not contain PHI and do not have potentially identifying information if further processed or combined
2) Controlled Data: Processed or Raw Data that represent a risk of re-identification and will undergo additional risk evaluation and approval steps prior to release
Data Management Tools
Brain-CODE features a comprehensive set of data management tools that allow researchers to securely upload, store and manage research data electronically. All tools can be conveniently accessed online through the Brain-CODE portal. In addition to providing a single point of access to data management tools, the Brain-CODE portal features private file repositories and discussions forums that researchers can use to facilitate sharing and collaboration.
For general inquiries or information about the portal, please e-mail help@braincode.ca
|
|
The Brain-CODE Subject Registry provides a secure way of entering personal health information from study participants. The Subject Registry is used to enter unique identifiers for enrolled subjects, as well as encrypt health card numbers. This allows research data to be integrated across modalities and securely linked with other health databases.
SUPPORT: help@braincode.ca |
|
|
OpenClinica is a commercial, regulatory-compliant clinical data management system for web-based electronic data capture of clinical assessment information collected from both clinical trials and clinical research studies.
SUPPORT: help@braincode.ca |
|
|
REDCap (Research Electronic Data Capture) is a regulatory-compliant and secure web application for building and managing online surveys, case report forms and academic research study databases.
SUPPORT: help@braincode.ca |
|
|
SPReD/XNAT is a comprehensive database for the storage, management, and analysis of imaging data including MRI, PET, EEG, CT, as well as other types of binary data such as gait measures and pathology.
SUPPORT: spred@braincode.ca |
|
|
LabKey enables tracking of genomics and other "omics" workflows, and managing and sharing resulting datasets.
SUPPORT: help@braincode.ca |
Training and Support
Training, tutorials, reference manuals and user support are available for all of the data management tools. Training may take the form of workshops, webinars or self-paced tutorials, depending on the system and the users' needs. For clinical data, standard electronic case report forms (eCRFs) are avalable to ID program researchers, including eCRFs for established Brain-CODE Common Data Elements (CDEs). In addition to eCRFs for CDEs, the Brain-CODE team can assist reserachers in developing and standardizing eCRFs for study-specific clinical assessments, and in providing authorized users with customized training and support on the data entry and data management requirements of their studies.
Accessing Data
Brain-CODE User Types
There are two general types of Users for Brain-CODE:
1) ID Program Investigators: OBI-funded investigators will have an account set up for them by Indoc and have access to data they have generated and from studies in which they are listed as a collaborator. Access to Controlled data they neither generated nor are collaborating on will require the completion of a Data Access Request and approval by the DAC.
2) External Users: This category would apply to all other users that are not funded by OBI. To acquire access, an account request must be made to help@braincode.ca. This process requires the applicant to provide their contact information and association, which are then verified. Access to any data that is not Public requires the completion of a Data Access Request and approval by the DAC.
Data Exclusivity
Data Exclusivity refers to the 12 month period during which data sets are solely accessible to study collaborators in Zone 1 and will not be available for data access requests by third parties. Data under exclusivity in Zone 1 will only be available to the collaborating research sites as indicated in the informed consent forms signed by participants. To enable data sharing beyond direct study collaborators, data will be processed and copied to Zone 2 after the period of exclusivity and made available to third-parties in Zone 3 as described in OBI’s Informatics Governance policies (available at https://www.braincode.ca/content/governance)
Data Access Committee (DAC)
The purpose of the Data Access Committee (DAC) is to develop data access and sharing policies, which address eligible users, data embargoes, acknowledgement and ethics requirements, and make recommendations to the Informatics Steering Committee regarding the release of data to third parties. Members of the DAC include representatives from research, neuroinformatics, and OBI.
Under no circumstances will the DAC recommend issuing a third party access to Brain-CODE controlled data without a valid approval from the third party’s REB. The DAC may also consider the scientific merit of the data access, including the assessment of a user’s affiliations and research hypothesis.
Data Access Requests
- The DAC will review Controlled Data access requests to ensure adherence to OBI’s Governance Policies as a whole, and any Applicable Laws and Guidelines, including PHIPA. The DAC will also examine the appropriateness of the data requested for its intended use. In collaboration with OBI and the Service Group, the DAC will make use of algorithms to analyze the risk that the release of any data requested, where appropriate, will lead to re-identification of study participants who have contributed data.
- The DAC will be responsible for verifying that only data which have been subjected to de-identification tools available and that has a minimal risk of re-identification, are disclosed.
- Data will be released on the condition that the external researcher signs a Data Use Agreement with applicable confidentiality requirements and provisions that prohibit any attempts to re-identify data.
- The DAC may then issue a recommendation to the Informatics Steering Committee to release the requested information.
- To the extent possible, data will remain within the Brain-CODE environment where any analysis or other related manipulations would take place, restricting the download of data.
Brain-CODE Operates Under Informed Consent
The expressed written consent of study participants, from whom data have been collected, is required to transfer data, which may include PHI, into Brain-CODE.
The Brain-CODE standard consent language must be used in studies that are conducted in collaboration with OBI and for which data will be uploaded to Brain-CODE.
Learn more about Brain-CODE consent language and other standards
Please contact governance@braincode.ca for additional standard consent templates and procedures for other participant ages.





